GHB, commonly known as G, is a central nervous system depressant consumed for its euphoric and empathogenic effects. While the compound has a few legitimate medical uses, beginning in the 1990s it acquired the reputation as both a club drug and date-rape drug. Prior to its federal scheduling in 2000, GHB was easily made from premeasured precursors (“GHB kits”) that were widely available on the internet. Today, illicit GHB is manufactured in clandestine settings from the strictly regulated precursor chemical, GBL. A simple reaction with a base such as sodium hydroxide or sodium bicarbonate readily converts GBL to the sodium salt of GHB. This article will overview the history of GHB manufacturing and the processes involved in its synthesis.
Where was GHB First Manufactured?
The first laboratory synthesis of GHB dates back to the late 19th century. It was first synthesized in 1874 by a Russian chemist named Alexander Zaytsev, via the reduction of succinyl chloride.
Extensive research on GHB’s pharmacological activity didn’t occur until the 1960s. In 1960, a French physician named Henri Laborit synthesized GHB as part of an investigation into GABA analogs that would cross the blood-brain barrier. A few years later, two American researchers named Samuel Bessman and William Fishbein discovered that GHB is an endogenous brain metabolite.
Not long after its properties were described, investigation into GHB began for its use as an anesthetic and hypnotic agent. It was withdrawn for this use, however, once clinical trials discovered that it produced serious side effects, such as seizures and convulsions. By the 1970s, GHB was being examined for medical conditions such as narcolepsy and alcohol/opiate withdrawal. GHB, as its sodium salt known under the brand name of Xyrem, is still prescribed for these purposes. One pharmaceutical company (Jazz Pharmaceuticals) manufactures the majority of it. In a clandestine setting, it is easily synthesized from precursor chemicals that are, however, increasingly difficult to obtain.
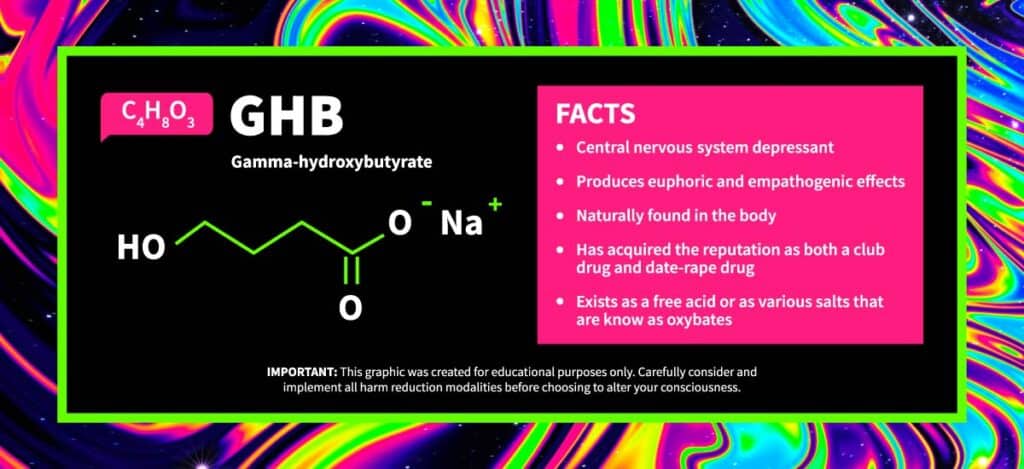
The GHB Molecule and Chemical Structure
GHB is a central nervous system depressant that is also found naturally in the body. In the brain, it acts as a putative neurotransmitter and precursor to the inhibitory neurotransmitter GABA (γ-aminobutyric acid).
Structurally, GHB is a simple short-chain fatty acid with a molecule formula of C4H8O3. At one end of the four-carbon chain is a carboxylic acid group (-COOH), and at the other end is a hydroxy group (-OH). This hydroxy group replaces an amino group in GABA’s chemical structure, otherwise, their chemical structures are identical. Unlike GABA, however, GHB can readily cross the blood-brain barrier to bind to GABAB and GHB receptors.
GHB exists as a free acid or as various salts that are known as oxybates. Most commonly, it is found as its sodium salt (sodium oxybate, brand name Xyrem), but its potassium salt (potassium oxybate) is also seen. Both of these salts are white solids that are highly soluble in water and alcohol.
1,4-Butanediol
1,4-butanediol is a prodrug of GHB, meaning it rapidly converts to GHB in the body by the action of an enzyme known as alcohol dehydrogenase. Structurally, 1,4-butanediol consists of a four-carbon chain with hydroxy groups (-OH) bound to each terminal carbon. In its pure form, it exists as a colorless, viscous liquid that is commonly found in industrial solvents. Owing to its higher availability, 1,4-butanediol is sometimes used to make GBL. It can be dehydrogenated into GBL when refluxed with a strong base and copper chromite.
GBL
GBL, or γ-butyrolactone, is the major precursor used in the illicit manufacturing of GHB. It also acts as a prodrug to GHB in the body, where lactonase enzymes convert it in the blood into GHB. GBL is a common ingredient in industrial solvents, such as paint strippers and floor cleaning products.
Structurally, GBL is a simple four-carbon ring of the tetrahydrofuran chemical class. This makes it a lactone of GHB (that is, a cyclic ester that is formed by the elimination of water). GHB and GBL exist in equilibrium and can transform into each other, depending on the pH of the solution. Under basic conditions, GBL is hydrolyzed to form GHB. Under acidic conditions, GHB cyclizes back into GBL.
GBL is a closely watched List I chemical in the United States, so it is commonly produced from other compounds. Tetrahydrofuran can be oxidized to produce GBL. Alternatively, GBL can be synthesized from GABA using the Sandmeyer reaction.
How to Make GHB: Key Procedures Explained
Illicit GHB can be easily synthesized in the kitchen with minimal organic chemistry experience. It is synthesized from its precursor GBL by changing the pH with the addition of a basic catalyst such as sodium bicarbonate or sodium hydroxide. The synthesis requires only a few steps and results in a single product, the sodium salt of GHB. GBL is difficult to obtain, so it is commonly synthesized from 1,4-butanediol or from GABA using the Sandmeyer reaction.

Dissolve Sodium Bicarbonate in Water
Using stoichiometric quantities, dissolve pure sodium bicarbonate (NaHCO3) in distilled water within a large glass container. Although sodium hydroxide can also be used, sodium bicarbonate is safer to handle, ingestible, and easier to obtain.
Slowly bring the solution to a boil while continuously stirring with a glass rod. As the solution boils, it will give off carbon dioxide as sodium bicarbonate breaks down into sodium carbonate (Na2CO3), a stronger base.
Slowly React GBL with the Basic Solution
Reduce the heat so the solution is slightly boiling, and then add GBL in small increments to the solution. Keep the solution at a light boil for at least thirty minutes. It can help to keep track of the temperature of the solution with an immersed thermometer, making sure it doesn’t go above 150°C. In this step, the GBL will be hydrolyzed to the desired hydroxycarboxylic acid (GHB).
Titrate the pH of the Solution to Neutral
After boiling for half an hour, check the pH of the solution with universal pH paper. The ideal pH for the reaction is close to neutral (7). If the solution is too alkaline (above 8), more GBL can be added while refluxing. When the reaction is complete, the solution will be completely colorless.
Purify the Final Product if It is Not Colorless
If the GBL contains impurities, the reaction can lead to a slightly yellow solution. This can be purified by adding activated charcoal to the solution, then boiling for ten additional minutes. After boiling, cool the solution and filter the charcoal away after washing it with distilled water. The solution will crystallize at a concentration roughly around 50% sodium GHB.
If a powder is desired, the solution can be heated to 150°C then poured onto a thin metal sheet, where it will cool and solidify. If kept in liquid form, dye the liquid with blue food coloring to distinguish it from drinkable liquids like water.
Disclaimer: The substance GHB is potentially illegal, and Neonjoint does not condone its manufacture or use in any context where it would be against the law. Providing this information is an important step toward educating the public about a substance that many people may come into contact with. The information in this guide should not be used as a replacement for seeking professional medical advice.


![[NJ] The Easiest Ways to Buy Weed in Buffalo A Full Guide (5)](https://neonjoint.com/wp-content/uploads/NJ-The-Easiest-Ways-to-Buy-Weed-in-Buffalo-A-Full-Guide-5-768x432.jpg)


